Sleepers Assignment Read and Respond # 06
- Update
- Open Admission
- Published:
Testing an early online intervention for the treatment of disturbed sleep during the COVID-xix pandemic in cocky-reported good and poor sleepers (Sleep COVID-nineteen): study protocol for a randomised controlled trial
Trials volume 22, Commodity number:913 (2021) Cite this article
Abstruse
Background
Theoretical models of indisposition suggest that stressful life events, such equally the COVID-19 pandemic, can cause acute insomnia (brusk-term disruptions to sleep). Early interventions may forbid short-term sleep bug from progressing to indisposition disorder. Although cerebral behavioural therapy for insomnia (CBT-I) is effective in treating insomnia disorder, this can exist time and resources-intensive. Further, online interventions can be used to deliver treatment to a big number of individuals. The objective of this study is to investigate if an online behavioural intervention, in the class of a leaflet, which has been successfully used alongside CBT-I for acute indisposition, can reduce symptoms of acute insomnia in poor sleepers.
Methods
A full of 124 self-reported good and poor sleepers volition be enrolled in an online stratified randomised controlled trial. After baseline assessments (T1), participants will complete a 1-week pre-intervention sleep monitoring period (T2) where they will consummate daily sleep-diaries. Poor sleepers (n = 62) will be randomly allocated to an invention or wait-listing grouping, where they will receive the intervention (T3), or volition exercise so after a 28-solar day delay. Good sleepers (n = 62) will be randomly assigned to an intervention or no intervention group. All participants volition complete a 1-week post intervention sleep monitoring period using daily sleep diaries (T4). Participants volition exist followed upwards at 1 week (T5), 1 month (T6) and three months (T7) mail intervention. The master outcome measure out will be indisposition severity, measured using the Indisposition Severity Index. Secondary outcome measures volition include subjective mood and subjective slumber continuity, measured using sleep diaries. Data volition exist analysed using an intention-to-care for approach.
Discussion
It is expected that this online intervention volition reduce symptoms of acute insomnia in self-reported short-term poor sleepers, and will also preclude the transition to poor sleep in good sleepers. We expect that this will demonstrate the feasibility of online interventions for the treatment and prevention of astute insomnia. Specific advantages of online approaches include the depression cost, ease of administration and increased availability of handling, relative to confront-to-face therapy.
Trial registration
ISRCTN43900695 (Prospectively registered 8th of Apr 2020).
Administrative information
Note: the numbers in curly brackets in this protocol refer to SPIRIT checklist detail numbers. The order of the items has been modified to grouping like items (see http://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/).
| Title {one} | Testing an early online intervention for the handling of disturbed sleep during the COVID-19 pandemic in self-reported adept and poor sleepers (Sleep COVID-xix): study protocol for a randomised controlled trial |
|---|---|
| Trial registration {2a and 2b}. | ISRCTN43900695; prospectively registered on viiithursday April 2020 |
| Protocol version {three} | Protocol version one.i (October 22nd, 2021). |
| Funding {4} | The nowadays study is funded by Northumbria University. |
| Author details {5a} | Greg J. Elder, Nayantara Santhi, Pamela Alfonso-Miller, Jason G. Ellis (Northumbria Sleep Research, Northumbria University, Newcastle upon Tyne, UK) |
| Name and contact information for the trial sponsor {5b} | Organization: Northumbria University, Contact Name: Samantha Male monarch, Contact Accost: Sutherland Building, Newcastle upon Tyne, NE1 8ST, Great britain Telephone: 0191 243 7108 Email: samantha.male monarch@northumbria.ac.united kingdom |
| Role of sponsor {5c} | The funding source and written report sponsor (Northumbria University) has had no part in the design of this study and will not take any part in the execution of the study. Furthermore, the sponsor will take no role in the analysis and interpretation of the report results, in the writing of the written report, or in the decision to submit the final report for publication. |
Introduction
Groundwork and rationale {6a}
Insomnia is very common and is defined as dissatisfaction with sleep quantity, sleep quality, or both, due to difficulties initiating and/or maintaining sleep, for at least three nights per week, for a period of at least iii months [one]. Within industrialised societies, approximately half-dozen–ten% of the population have indisposition, where prevalence rates have increased in contempo years; additionally, upwards to 48% of the population report the presence of indisposition symptoms [two, iii]. Therefore, insomnia is a highly prevalent problem. Insomnia disorder (across 3 months) is associated with a significant economic burden [4] and is a risk gene for a range of concrete wellness weather including hypertension, cardiovascular diseases and psychological weather including depression [5,six,7].
Theoretical models of insomnia (e.g. Spielman'southward "3P" model) suggest that psychophysiological arousal caused by a stressful life upshot can crusade a brusque-term disruption to slumber (i.e. acute insomnia) [8, 9]. Over time, this can result in maladaptive compensatory behaviours, such equally spending excessive time in bed or becoming preoccupied with sleep, which consequently creates a long-term problem of poor slumber through behavioural workout [10]. Acute indisposition is mutual, where the annual incidence rate is potentially every bit high as 27 to 37% [11, 12]. One study has demonstrated that approximately 7% of individuals with acute insomnia subsequently go on to develop insomnia disorder, and a further 20% of individuals demonstrate variable sleep disturbances and may proceed to develop insomnia disorder, albeit at a slower rate [12]. Given the associated private and economic health brunt associated with insomnia disorder, strategies which forestall the transition from acute to chronic indisposition are important.
Previous naturalistic studies have indicated that stressful events, in the course of natural disasters such as earthquakes or hurricanes, or events such every bit war, can disrupt sleep [13,14,xv,16]. The ongoing COVID-19 pandemic may represent one such stressful life event. A recent meta-analysis has demonstrated that the global prevalence of sleep problems during the COVID-nineteen pandemic is high, where approximately 40% of the general population and healthcare workers are affected by sleep disturbances [17]. Individual fear of infection, or perceived infection severity, may also represent a stressor in the context of the COVID-19 pandemic. For example, one cross-sectional written report from China demonstrated that sleep disturbances were common, and people who believed that COVID-19 had caused a college number or deaths or that COVID-19 was non easy to cure, were more likely to experience sleep disturbances [eighteen]. Additionally, one Italian study has indicated that as well equally poor sleep quality beingness very mutual, individuals who had a greater fear of direct contact with people infected past COVID-19, and those with an uncertain COVID-nineteen infection status, had an increased risk of developing sleep disturbances, and higher anxiety and distress [19]. Therefore, COVID-nineteen slumber disturbances are extremely probable, and early interventions may present an opportunity to prevent a curt-term sleep disruption from becoming a long-term clinical sleep problem [20].
Pharmacological treatments, such as benzodiazepines, are often used in the management of insomnia and can exist effective treatments in the brusque term [21]. Withal, pharmacological agents are associated with a range of side effects and adverse outcomes, including drowsiness, tolerance, dependency and negative impacts upon next-day cognition, in improver to increased mortality and suicide risk [21,22,23,24]. In particular, the use of pharmacological agents is particularly problematic in older adults [25]. Therefore, non-pharmacological alternatives are necessary.
Ane not-pharmacological treatment, which is highly constructive in the treatment of chronic indisposition, is cognitive behavioural therapy for insomnia (CBT-I) [21]. CBT-I is a structured psychotherapy with the aim of identifying and changing maladaptive cognitions and behaviours which contribute to the maintenance of insomnia [26]. CBT-I results in equivalent improvements to those observed using pharmacological treatments, with the benefit of being more than durable (compared to pharmacological treatment discontinuation) and concomitant reductions in symptoms of anxiety and depression [21]. For these reasons, CBT-I is recommended equally a starting time-line treatment for chronic indisposition [21]. Withal, the widespread delivery and uptake of CBT-I is prevented by the lack of qualified providers and loftier attrition levels [20]. Therefore, traditional CBT-I may be as well time- and resource-intensive to be feasible and applied in the treatment of acute insomnia, and shorter interventions are likely to exist of benefit.
One previous study institute that the apply of a cocky-help leaflet (based on stimulus control, cognitive control and imagery distraction techniques), delivered alongside a 60–70 min single ("ane shot") session of face-to-face CBT-I for astute insomnia, effectively reduced insomnia severity [20]. Furthermore, follow-up studies have demonstrated effectiveness when the leaflet has been used alongside CBT-I treatment, in a grouping format and in a male adult prison house population [26, 27]. Internet-based interventions can exist used to evangelize treatment to more individuals than contiguous therapists, with lower relative costs [28]. Therefore, this self-help leaflet is well-suited to an online delivery model and tin can be used to accomplish a large number of people in the context of a large-scale stressful issue. Indeed, internet-based CBT-I has been shown to be constructive, with similar effect sizes to confront-to-face treatments [29]. In farther support of an online delivery model, recent studies take as well demonstrated that sleep extension does not occur in the context of astute insomnia [30, 31]; therefore, incorporating sleep restriction is not necessary. This intervention may also aid the prevention of sleep problems in individuals with proficient sleep, where the stress of a naturalistic event can nonetheless cause sleep disturbances [thirteen,14,fifteen,16]. Therefore, this study will examine if an online self-help leaflet is effective in reducing symptoms of acute insomnia in poor sleepers. This study will also examine how long the effects last for at follow-up stages, and finally, investigate if the leaflet can prevent the development of poor sleep in good sleepers.
Objectives {7}
The primary objective of this written report is to examine the effectiveness of an online intervention for poor slumber in the context of an ongoing stressful major life event. This volition be implemented by assessing if this intervention tin reduce indisposition severity in the short term (1 week mail service intervention follow-up) and the long term (i- and three-month mail intervention follow-ups). It is hypothesised that the intervention volition reduce insomnia severity in poor sleepers.
Furthermore, the secondary objectives of this study are to assess if the intervention can (one) reduce subjective anxiety and depression in good and poor sleepers, (2) appraise if the intervention can improve sleep continuity (derived from subjective sleep diaries) in good and poor sleepers and (3) examine if the intervention can prevent the development of acute insomnia in good sleepers. It is hypothesised that the intervention will reduce subjective symptoms of anxiety and depression in proficient and poor sleepers, and prevent the development of astute indisposition in good sleepers, as reflected by lower indisposition severity scores in those who have received the intervention.
Trial design {eight}
This study is designed as a stratified randomised controlled trial involving both self-reported skillful and poor sleepers. Good sleepers volition be randomised into an intervention or no intervention group using a 1:1 allocation ratio. Poor sleepers will be randomised into an intervention or wait listing (where participants will receive the treatment after a 28-day delay) grouping with a 1:1 allotment ratio. Overall, skilful and poor sleepers will participate in the study at a 1:1 ratio (Fig. one).
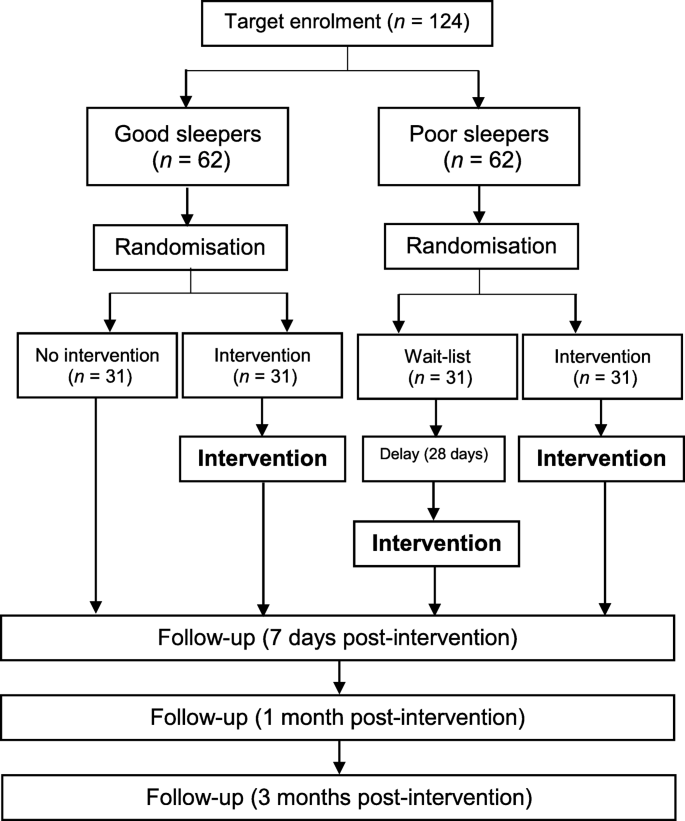
Participant flowchart (NB: skillful sleeper participants who do not receive the intervention will complete the follow-up stages at an equivalent time indicate)
Methods: participants, interventions and outcomes
Study setting {9}
Participants will complete all study procedures completely online, and this volition include the delivery of the study intervention. There are no geographical restrictions upon participation.
Eligibility criteria {10}
Both self-reported healthy adept sleepers, who practice non study any sleep problems, and individuals who report electric current sleep problems, where they accept experienced sleep problems or betwixt 2 weeks and iii months at the point of written report entry, will be eligible to participate.
Inclusion criteria
Individuals who are good and poor sleepers are eligible to participate if they meet the following criteria: (i) they are aged 18 years or above; (ii) if they consider themselves to accept a sufficient level of English language comprehension to be able to sympathize and consummate all report measures.
Participants who are self-reported poor sleepers must meet the criteria for acute insomnia, as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-v [i];). Specifically, participants must report (1) difficulties in falling asleep, staying comatose, or enkindling too early for at least iii nights per week, for a fourth dimension period of between ii weeks and 3 months, and (2) distress or impairment due to the slumber loss. Both criteria must occur despite the individual having had an adequate opportunity for sleep.
Exclusion criteria
Participants cannot take role if they (1) report having chronic sleeping problems that persist more than than 3 months immediately prior to consent, (2) are actively seeking handling for their sleep problem, (3) have a cocky-reported history of head injury or (four) have had a diagnosis of schizophrenia, epilepsy or personality disorder, every bit the intervention involves lark techniques which may increase rumination and after influence the effectiveness of the slumber intervention.
Who will take informed consent? {26a}
Participants will provide informed consent, which volition be recorded electronically.
Additional consent provisions for collection and use of participant information and biological specimens {26b}
Participants will exist asked for specific boosted consent to allow for their anonymised data to exist combined and used in similar studies, in order to maximise the scientific value of the data. Withal, participants can take role in this study without consenting to the re-employ of their data. Consent for biological specimens is non applicable, as biological samples are non beingness nerveless.
Interventions
Explanation for the option of comparators {6b}
The behavioural leaflet that will exist implemented in this study has previously been successfully used to reduce insomnia symptoms (measured on the basis of ISI scores) in individuals with indisposition and acute insomnia [20, 26, 27]. In line with our previous studies, we will compare insomnia severity earlier and after the intervention [twenty, 26, 27]; this is appropriate given that the chief aim of this study is to examine the brusque-term effectiveness of this intervention in poor sleepers. A wait-listing grouping of poor sleepers, who will receive the intervention after a 28-day filibuster, will be too used to examine drop-out rates in order to inform the apply of wait-listing control designs using this intervention in future. This blueprint is ethically advisable, as look-listing poor sleepers will have therefore also the opportunity to receive the intervention.
Good sleeper comparators will exist those who do, and practice not, receive the intervention. This blueprint is advisable given the aim of the report.
Intervention description {11a}
Participants, who are eligible to receive the intervention, volition be provided with an online two-page slumber cocky-assistance leaflet in the format of a PDF file. Participants will be specifically encouraged, and permitted, to download, save, physically print or have mobile telephone screenshots of the leaflet.
The intervention is an online version of a self-help leaflet which was used in a previous study [twenty]. This self-help leaflet outlines the principles of Stimulus Control, Cognitive Control and Imagery Distraction techniques [32, 33]. Specifically, the self-help leaflet aims to improve slumber by identifying and addressing sleep-related dysfunctional thinking. This is done by providing education about sleep, techniques to distract from intrusive worrisome thoughts at night, and guidelines for slumber-related stimulus control. These rules to improve slumber are presented in the format of the "three D's": "Detect", which provides individuals with instructions for completing a sleep diary, "Disassemble" which provides stimulus command instructions, and "Distract", which refers to cerebral control and imagery distraction instructions [20].
Criteria for discontinuing or modifying allocated interventions {11b}
At that place are no special criteria for discontinuing or modifying the allocated interventions. As the intervention will be self-administered, participants will be complimentary to discontinue the treatment whenever they wish to. Similarly, there are no restrictions on the subsequent use of the intervention leaflet.
Strategies to ameliorate adherence to interventions {11c}
Participant treatment adherence will be formally monitored by verifying that individuals have downloaded or viewed the slumber intervention leaflet. Adherence will exist recorded electronically. In that location are no specific strategies that will exist used for adherence improvement in this trial.
Relevant concomitant care permitted or prohibited during the trial {11d}
There are restrictions on pharmacological or non-pharmacological concomitant care earlier and during report participation. This is necessary to verify whether the intervention can reduce symptoms of acute insomnia. However, as the study will be delivered entirely online, the employ of concomitant care will not be formally monitored. Furthermore, individuals who are actively seeking treatment for their sleep bug, irrespective of how long they have had the sleep trouble, will not exist able to participate in the study.
Provisions for mail-trial care {30}
The present study has a minimal gamble of side effects and therefore at that place is no provision for post-trial care. However, if any practiced sleeper participants, who have not received the intervention, report the development of short-term sleep problems during the written report, the online intervention will be offered to them costless of charge. This study is very unlikely to cause any psychological distress, as the intervention is a safe and established handling that has been successful in previous studies in our inquiry group.
Participants will be directed to their general practitioner (GP) if they are concerned about their physical or psychological health equally a issue of taking part in the study. Northumbria Academy has insurance to embrace non-negligent damage associated with the study.
Outcomes {12}
Chief outcomes
The primary result measure is the Insomnia Severity Index (ISI [34];). This volition be assessed at baseline, immediately prior to the intervention, and 1-week, ane-month and 3-month mail service intervention.
Secondary outcomes
Secondary result measures include subjective mood and sleep continuity, as follows:
- (i)
Changes in the 7-item Generalised Anxiety Disorder Questionnaire (GAD-7 [35];)
- (2)
Changes in the 9-particular Patient Wellness Questionnaire (PHQ-9 [36];)
- (3)
Changes in subjective sleep continuity, measured using the Consensus Sleep Diary (CSD-Yard [37];). The following variables volition be assessed: number of awakenings (NWAK), wake later on sleep onset (WASO), full sleep time (TST), sleep onset latency (SOL) and sleep efficiency (SE%).
Subjective sleep continuity volition exist compared pre-intervention and post intervention (i.due east. in the week before and after the intervention).
Participant timeline {xiii}
Figure two displays the participant timeline.
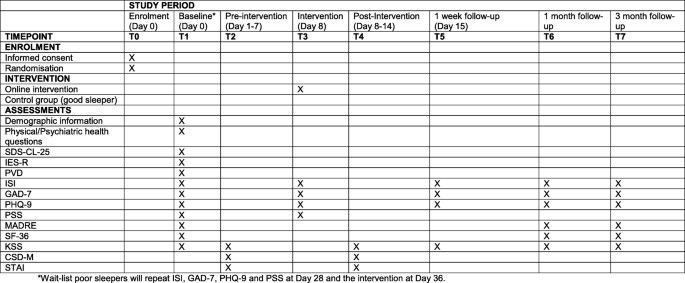
SPIRIT figure and overview of enrolment, interventions and assessments
Sample size {14}
A total sample size of 124 is required for the present study. This is based on an a priori power analysis which was conducted using Thou*Power 3.1 [38]. It is expected that in that location volition be an overall drop-out rate of fifteen% (n = xvi) during the study, and therefore, a full sample size of 124 is required. An equal number of participants will be recruited to each group (n = 31). Proficient and poor sleepers will be recruited at a one:i ratio (62:62 participants)
A minimum of 54 poor sleeper participants are required to appraise the master outcome and meet the study'southward objectives on the basis of an expected medium result size (d z = 0.50) at 95% ability for the main outcome mensurate. Our research grouping has previously observed medium-to-large result sizes (d = 0.64) when the printed version of this intervention has been used in people with insomnia disorder [20], and large effect sizes (d z = two.35) in prison inmates with acute insomnia [26], both upon the Indisposition Severity Alphabetize equally the primary upshot. Additionally, a contempo meta-analysis of cyberspace-delivered cerebral behavioural therapy for insomnia observed large consequence sizes upon insomnia severity (g = 0.89 when adjusted for publication bias; unadjusted chiliad = ane.09) [29]. Therefore, on this ground, a medium event size is expected for the nowadays written report. A minimum of 54 good sleeper participants are required to appraise the master event on the basis of an expected medium effect size (f 2 = 0.25).
For poor sleepers, the a priori power assay was calculated on the footing of the primary outcome measure out (ISI) being compared between baseline and 1-week follow-upward for poor sleepers, using a 2-tailed repeated-measures t-test (d z = 0.50; 95% power). For good sleepers, the a priori ability analysis was calculated on the basis of the principal consequence measure out existence compared using a 2 (group) × 2 (time point: baseline vs. ane-calendar week follow-upwardly) mixed analysis of variance (ANOVA), with a medium event size (f two = 0.25).
Recruitment {xv}
Participants will be predominantly recruited online. The study URL will be placed online and publicised to potential participants using the Northumbria University website and various social media channels, including the Northumbria University Twitter and Facebook platforms. The URL volition also be included on the ISRCTN trial registration page. Participants will not receive any financial incentive and the intervention will exist offered to individuals free of accuse.
Assignment of interventions: allocation
Sequence generation {16a}
The allocation sequence and randomisation will be automatically generated using the survey software (Qualtrics). Skilful sleepers (n = 30) will be randomly allocated using a 1:1 ratio into either an intervention or no-intervention group. Poor sleepers (north = thirty) will exist randomised into an intervention or look-list group, with a 1:ane resource allotment.
Concealment machinery {16b}
All participants who fulfil the inclusion criteria, and provide informed consent, will be randomised and given a unique identification number in Qualtrics prior to completing the baseline assessments. Qualtrics will automatically allocate participants to the appropriate study condition and section after they accept entered the study and indicated whether they are a good or poor sleeper. Darkening will therefore be assured, equally members of the enquiry team volition be completely unable to determine which arm of the study good, or poor, sleepers take been allocated to until after report entry.
Implementation {16c}
The resource allotment sequence will exist automatically generated past the Qualtrics software without any influence from whatsoever member of the research team. Participants will be allocated a numeric grouping identification lawmaking in the written report dataset to denote group allocation, equally follows: 1 = skilful sleeper (no intervention); 2 = expert sleeper (intervention); 3 = poor sleeper (wait listing) or 4 = poor sleeper.
Assignment of interventions: blinding
Who will be blinded {17a}
Due to the nature of the intervention, participants volition exist aware of which condition they have been allocated to, and then they cannot be blinded to the report. Yet, the member of the enquiry team who will be conducting the statistical assay will be blinded to the written report condition until all data analysis has been undertaken. Any routine data monitoring will exist undertaken by a member of the research team who will non be responsible for conducting the statistical analysis.
Procedure for unblinding if needed {17b}
Participant-level code breaks volition only occur in emergency circumstances, where the cognition of the group assignment is judged to be clinically essential for the management of the individual. However, the take chances of this situation occurring is considered to exist extremely depression, given the safety profile of the intervention, and it is not expected that this will be necessary during the trial. In a situation where an emergency code pause is judged to be essential by the principal investigator, an independent member of the written report team, who will not accept the responsibility for undertaking statistical analyses, will be permitted to break the blinding past accessing the stored dataset. If a lawmaking break does occur, the principal investigator will maintain the blind equally much every bit possible. Whilst the allocation will be known to the participant, in all circumstances, the allocation will not be disclosed to any other study personnel. The written or verbal disclosure of the code will simply be washed where this is clinically necessary. All code breaks volition be documented.
Information collection and management
Plans for assessment and drove of outcomes {18a}
The unabridged written report volition be conducted online. The total listing of assessments and time points of completion are presented in Fig. two and are described beneath in more detail.
In addition to the intended master and secondary result measures, participants will complete measures of full general wellness status, subjective stress, sleepiness and dreaming. This will facilitate subsequent exploratory data analyses, either lonely or in combination with data from other studies. This is because, for case, these measures are potentially altered by insomnia (eastward.g. in the case of dream content [39, 40]), or because they might be involved in causing slumber disturbances (e.thousand. stress [41]).
Demographic and concrete/psychiatric wellness information
Participants will be asked brief demographic questions, including their educational level, month and year of birth, and occupational information (due east.g. their current task title, employment sector and function). Participants will also be asked whether or not they were furloughed (i.due east. that they were placed on a paid temporary leave of absenteeism from work by their employer), or were made unemployed during this menstruation. Participants volition be asked for a very brief self-reported physical and psychiatric medical history, including a list of current medication. Participants volition too be asked specific yes/no questions in relation to COVID-xix. This will include whether they accept had a examination for, a diagnosis of, or take demonstrated symptoms of COVID-19.
General health status
In order to characterise the general health condition and wellness-related quality of life of participants, they volition complete the 36-Detail Short Form Survey Instrument (SF-36 [42];). This assesses eight domains, including physical part, function limitations because of concrete health issues, actual pain, general wellness perceptions, general mental health, role limitations considering of mental health problems, social functioning and vitality. These are used to generate SF-36 scores ranging from 0 to 100, where higher scores correspond better part. The SF-36 has practiced reliability and validity [43].
Sleep
Participants will complete the post-obit slumber, sleepiness and sleep-related measures:
Sleep Disorders Symptom Checklist-25 (SDS-CL-25 [44];)
This is a measure of the presence or absence of six common slumber disorders (insomnia, obstructive sleep apnea, restless legs syndrome/periodic limb movement disorders, circadian rhythm sleep-wake disorders, narcolepsy or parasomnias). Individuals are provided with a list of 25 symptom statements (e.g. "I am tired, fatigued or sleepy during the twenty-four hours") and are asked to indicate the frequency of the item symptom. The SDS-CL-25 provides an indication of severity and morbidity of a item sleep disorder and is used in the present study every bit a research screening tool to assess the presence of any sleep disorder.
Insomnia Severity Alphabetize (ISI [34];)
The ISI is a seven-particular measure of insomnia, which assesses the nature, severity and result of indisposition. The ISI provides scores ranging from 0 to 28, where high scores represent more severe indisposition. Whilst originally, a cut-off score of ≥ 8 is used to place subclinical insomnia from an absence of insomnia, a cutting-off score of ≥ ten will exist used since this is optimal for identifying insomnia caseness in community samples [45]. As per previous studies [xx], the ISI volition be modified from the original measure to assess insomnia severity during the previous calendar week, as opposed to the preceding calendar month, in line with subjective sleep diaries. The ISI is a reliable and valid measure for quantifying perceived insomnia severity [34].
Consensus Slumber Diary (CSD-M [37];)
The CSD-M is a subjective sleep diary which will exist used to measure out sleep over a period of vii continuous days. Sleep continuity measures, including total sleep time (TST), time in bed (TIB), number of awakenings (NWAK), wake later on sleep onset (WASO), sleep onset latency (SOL) and sleep efficiency (SE%) volition be derived from completed diaries.
Karolinska Sleepiness Calibration (KSS [46];)
The KSS is a measure of subjective sleepiness, where sleepiness in the previous 10 minutes is rated using a 9-point scale, where college scores represent a greater level of sleepiness. The KSS has skillful validity [47].
Mannheim Dream Questionnaire (MADRE [48];)
The MADRE is used to measure the frequency, content and emotional tone of dreams, in addition to nightmare frequency and distress. The MADRE has high levels of test-retest reliability [48].
Psychological measures
Participants will complete the post-obit measures:
Spielberger Country-Trait Anxiety Inventory (STAI [49];)
The six-particular curt-class version of the state calibration volition be used to assess situational (state) anxiety symptoms, at the same time equally sleep diary information. The STAI asks participants to indicate how they are feeling at that item betoken in time, by indicating their level of agreement with a given statement (e.1000. "I experience calm") to which they reply using a Likert scale. Higher scores correspond more state anxiety. The STAI has adept reliability and validity and is sensitive to the individual changes in state anxiety [49].
Generalised Anxiety Disorder Questionnaire (GAD-7 [35];)
The GAD-seven is a seven-item questionnaire which assesses subjective anxiety symptoms, providing a score of between 0 and 21, where higher scores represent more severe anxiety. The GAD-7 has skillful validity and reliability [fifty].
Patient Health Questionnaire (PHQ-ix [36];)
The PHQ-9 is a nine-particular questionnaire which assesses subjective depression severity and provides a score of betwixt 0 and 27, where higher scores represent more severe depression. The PHQ-9 has high validity and reliability [51].
Perceived Stress Scale (PSS [52];)
This measures the extent to which life situations in the preceding month are perceived as being stressful and consists of xiv items. Total possible scores on the PSS range from zippo to 56, with higher scores representing higher levels of perceived stress. The PSS has good validity and reliability [53, 54].
COVID-19 infection susceptibility and distress measures
Participants will be asked to complete two measures in relation to COVID-19, where they will be asked to indicate their levels of subjective distress in relation to the pandemic, as well as their perceived infection susceptibility.
The Impact of Consequence Calibration-Revised (IES-R [55];)
The IES-R is a 22-item self-report measure which quantifies subjective distress caused by a specific stressful or traumatic life event, by asking participants to indicate how distressed they were with regard to specific difficulties in the previous calendar week. The IES-R provides a total score ranging from 0 to 88, where higher scores represent greater distress.
Perceived Vulnerability to Affliction questionnaire (PVD [56];)
The PVD is a fifteen-item self-report measure which assesses perceived susceptibility to infectious diseases and assesses emotional discomfort in situations with the potential for high infection transmission, with two separate subscales (Germ Aversion and Perceived Infectability).
Plans to promote participant retention and complete follow-up {18b}
To promote participant retention, participants volition receive automatic e-mail reminders at each stage of the study. Participants will also be encouraged to contact the study squad if they require back up during the trial.
Data direction {19}
All study data will be electronic and volition be obtained directly from Qualtrics. Participant informed consent will be provided electronically and all CRFs and associated documentation will exist electronic. The online component of the written report, and resulting dataset, will be extensively tested and checked prior to the first participant enrolment, to ensure that all methods of data entry are reliable. Furthermore, the full dataset volition exist checked and verified for integrity and quality at regular intervals during the study. This will also be done at the stop of the study by the research team. Any necessary changes to the dataset will be fully documented using a written log. Throughout the trial, anonymised information, relevant documentation and CRFs will be stored on secure, password-protected computer storage that will just be accessible to the research team and will exist backed up regularly. At the stop of the study, information will be exported to advisable statistical analysis software.
Confidentiality {27}
In order to protect participant confidentiality, all personal data will exist regarded as being strictly confidential and the written report will comply with the requirements of the Full general Information Protection Regulation (GDPR). Participant electronic mail addresses will be used for the sole purpose of sending participants reminders to complete their daily sleep diary, and at advisable follow-up stages of the study. Participant electronic mail addresses will be deleted upon study completion. Anonymised data volition be stored on secure, password-protected computer storage that will be accessible but by authorised members of the research squad. All electronic information will be stored in line with standard Northumbria University retention guidelines and in accord with all other relevant legislation (e.thou. GDPR).
In order to maximise the scientific value of the dataset, we intend to combine anonymised data from this study with data from similar studies conducted inside this research group, and where possible, larger collaborative studies. Provision has been made in the participant consent grade to allow for this and participants will exist permitted to opt out of this if they wish to do so.
Plans for collection, laboratory evaluation and storage of biological specimens for genetic or molecular analysis in this trial/futurity utilize {33}
This is non applicable: biological samples will not collected as part of this trial.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
Statistical analysis is pre-specified in the protocol and will be conducted either at the terminate of the study, or when the recruitment target is met. Statistical analyses will be conducted by a member of the team who will not accept any part in monitoring the information during the study. In order to meet the aims of the study, the statistical analysis will assess poor and adept sleepers separately.
In poor sleepers, the short-term effectiveness of the primary effect measure (ISI) volition be analysed by comparing pre/post intervention ISI scores, at baseline (solar day 28 for waitlist poor sleepers) and i-week follow-up, using a repeated-measures t-test. The longer-term effectiveness of the master upshot measure will be assessed by comparing baseline, 1-week, one-month and 3-month follow-up time points, using a one-way assay of variance (ANOVA) with an expected main event of time. A significant chief outcome will be followed up using post hoc tests every bit appropriate. Non-parametric equivalent tests volition be used if appropriate. Effect sizes (d z or partial eta squared) will be used to demonstrate effectiveness. Secondary event measures (GAD-7, PHQ-9) volition exist assessed in the same manner, with the exception of CSD-G sleep continuity variables, which volition exist compared pre and post intervention using repeated-measures t-tests, where sleep continuity p values will exist adjusted for multiple comparisons. The drib-out rate of the wait listing poor sleepers will also be examined and will be expressed as a number and every bit a percentage of participants, in order to inform the design of futurity trials.
In skilful sleepers, the short-term effectiveness of the intervention will exist assessed by comparing good sleepers who have, and have not, received the intervention, using a 2 (group) × 2 (time bespeak: baseline vs. 1-calendar week follow-up) mixed ANOVA with an expected significant interaction, which will exist followed upward using mail service hoc tests. The longer-term effectiveness will be examined using a 2 (grouping) × 4 (time point: baseline vs 1-week vs. 1-month vs. 3-calendar month follow-upward), with an expected significant interaction, which will be followed upwardly using mail service hoc tests. Issue sizes (partial eta squared) will exist used to demonstrate effectiveness. Secondary outcome measures (GAD-vii, PHQ-9) will be assessed in the same mode, with the exception of CSD-1000 sleep continuity variables, which will exist compared pre and postal service intervention using repeated-measures t-tests, where slumber continuity p values volition be adjusted for multiple comparisons.
Remission rates will exist calculated on the basis of a score of ten or more on the ISI at follow-up and every bit a pct of groups. In the good sleeper participants, those who transition to later having indisposition will exist divers as doing so on the basis of an ISI score of < ten points at baseline, and > 10 points at follow-upwards stages.
Acting analyses {21b}
Interim analyses are not planned.
Methods for boosted analyses (e.k. subgroup analyses) {20b}
No other additional analyses are planned.
Methods in assay to handle protocol not-adherence and any statistical methods to handle missing information {20c}
Information will exist analysed using an intention-to-treat approach. Large participant drib-out rates are not anticipated and any expected driblet-outs are factored into the target sample size. The primary outcome analysis will be based simply on the complete case/observed outcomes, and imputation of missing data will non take place, since there is no reason to believe that participants who may exist lost to follow-up will occur randomly.
Plans to give access to the full protocol, participant-level data and statistical code {31c}
The dataset, protocol and statistical lawmaking (if applicable) will exist made available to appropriately qualified investigators upon reasonable asking to the principal investigator. Our intended policy is that the research team will accept sectional employ of the information for a menses of 12 months from the end of the projection, or until the data is published, if this is required aslope publications.
Oversight and monitoring
Limerick of the coordinating heart and trial steering committee {5d}
As this is a study with an extremely low risk of side effects due to the behavioural nature of the intervention, a formal Trial Steering Committee is not required. The trial volition be managed and monitored locally past a study management group consisting of the named investigators, who will meet on a fortnightly basis. Quality command will be maintained through adherence to all relevant Northumbria University standard operating and research governance procedures and the principles of Skillful Clinical Practice.
Composition of the information monitoring commission, its function and reporting structure {21a}
A Information Monitoring Committee is not required in this report due to the intervention having only an extremely low gamble of side effects.
Adverse event reporting and harms {22}
This is a low-risk trial. However, the protocol does have provision for adverse events (AEs), where all AEs will be reported and categorised as to expectedness, relatedness and severity. The severity of all AEs will exist graded on a iii-bespeak scale of intensity (balmy, moderate, severe). For the purposes of this study, whatsoever adverse events (AEs) which the investigators are notified of during the report (i.e. from report entry to the final report measure) will exist recorded. If an AE occurs, the research team volition have access to all information accrued to that bespeak and will have the power to terminate the trial early.
Frequency and plans for auditing trial conduct {23}
Routine trial bear will exist monitored by the enquiry team. The inquiry team will permit the relevant Northumbria University ethics committee, and sponsor, to conduct study-related monitoring and audits, when this is specifically requested. The research team volition provide direct access to source data and report documentation, if required.
Plans for communicating important protocol amendments to relevant parties (e.chiliad. trial participants, ethical committees) {25}
Whatever modifications to the protocol which may touch on upon the comport of the written report (eastward.yard. the report objectives, blueprint, population, proposed sample size or procedures) will require a formal amendment to the protocol, and an upstanding amendment. Modifications will be formally documented on the nigh recent version of the study protocol, where a summary of changes will exist provided. The ISRCTN record volition also be updated.
Dissemination plans {31a}
Information technology is predictable that the overall results of this study will be submitted to an advisable peer-reviewed publication within 12 months of written report completion. The results of the study will also be presented at relevant national and international scientific meetings. Participants volition be able to asking a summary of the study results upon completion of the study, and we volition aim to provide a summary of findings on the Northumbria Academy website.
Discussion
The primary objective of this online randomised controlled trial is to investigate the effectiveness of a web-based behavioural intervention for poor sleep in the context of the ongoing COVID-xix pandemic. It is expected that this intervention volition reduce insomnia severity in the short term, and long term, in poor sleepers who receive the intervention. Secondary objectives of this trial will include assessing if the intervention tin amend subjective anxiety, depression and sleep continuity in poor sleepers, and if the intervention tin prevent expert sleepers from developing acute insomnia. It is expected that the intervention volition improve subjective feet, low and sleep continuity in poor sleepers and that the intervention volition foreclose the transition from adept sleep to acute insomnia.
If, as expected, the behavioural intervention is effective, this report volition accept a number of implications. Whilst we have previously successfully used this intervention alongside contiguous CBT in a range of populations [20, 26, 27], disadvantages of this approach tin include the requirement of trained personnel and the associated time and costs. Specific advantages of an online intervention approach include the depression toll and ease of administration, and relative scalability, since online interventions can potentially reach more than individuals with insomnia complaints than contiguous therapists, and this fashion of treatment delivery is generally adequate to individuals [28, 57]. This is besides price-effective, since cyberspace-based CBT-I tin can be used to deliver therapy to a greater number of individuals than would exist possible in-person using the aforementioned number of practitioners [28]. Additionally, online interventions have advantages in the context of COVID-nineteen, since these interventions can exist delivered without accommodating for social distancing measures, or fear of contamination, which may exist problematic or require additional resource [58]. If feasible and constructive, this would exist useful in terms of both the prevention and treatment of acute indisposition. This is very likely to have subsequent economical benefits, both in terms of prevention and handling: poor sleepers have an economic cost to society which is 10 times greater than practiced sleepers [4]. This written report may also have implications for hereafter astute insomnia trial design. To date, no studies take examined the long-term effectiveness of this behavioural intervention for acute insomnia, when it has been delivered online. It is possible that to retain any benefits, in skillful and poor sleepers, "booster" behavioural interventions, or boosted back up, may be required [xx], and this could be examined in future trial designs.
This written report is likely to have four main limitations. Firstly, although the report has been designed with an predictable drib-out charge per unit of 20%, show from other online behavioural interventions has shown that the drop-out rate may exist higher when CBT interventions are delivered online instead of in-person [59]. This too appears to be problematic in the context of insomnia research, where attrition rates may exist as high as 50% [57]. Secondly, a further potential limitation is that the provision of boosted support may exist necessary to maintain effectiveness. For case, evidence from cyberspace-based CBT for depressive and anxiety symptoms indicates that support tin can increase adherence and the subsequent effectiveness of the treatment program [60], and this is besides the instance in insomnia treatment when it is delivered online [61]. All the same, ane limitation of providing back up is that this may require additional cost and staffing resources, since the provision of human feedback typically requires 15–thirty min of work per participant per handling session [28]. Equally an alternative, automated methods of feedback may be effective whilst retaining the low costs of an online delivery model [28].
Thirdly, it is best-selling that a further limitation of the study is in the open-label design, and the fact that the primary endpoint is participant-reported, which has the potential to result in detection bias or cess bias [62]. However, the Insomnia Severity Index, which is existence used every bit the principal endpoint, is a reliable and valid musical instrument of detecting indisposition in the population and is sensitive to treatment response [45]. Whilst the choice of outcome measure out is therefore appropriate given the aims of the present study, futurity trials may wish to address these potential limitations.
Finally, it is not possible to formally monitor treatment adherence in the nowadays report. We have tried to mitigate the lack of formal monitoring past electronically verifying that participants have viewed the intervention leaflet, and by providing instructions which specifically encourage participants to download, save and impress the behavioural leaflet and to refer to this whenever they wish to practice and then. However, it is still possible that there will be a large variation in adherence, where some participants may only use the leaflet for a very curt period of time, and other participants are probable to refer to this on a regular footing. These potential limitations are probable to be outweighed by the fact that the nowadays study pattern will provide an indication of the effectiveness of internet-based behavioural interventions for astute insomnia in large-scale, representative, "real-globe" situations [57].
Overall, there is a clear demand for treatments which prevent the transition from acute to chronic indisposition, given the associated health and economic brunt associated with insomnia. If constructive, spider web-based behavioural interventions which prevent this transition will be of benefit. Additionally, interventions which prevent good sleepers from developing sleep disturbances during stressful naturalistic events are too likely to exist of private and societal benefit, and one specific reward if they are shown to be effective, is that they tin exist very quickly rolled out to targeted populations.
Trial status
The current version of the written report protocol is Version 1.ane (date: October 22, 2021). The study is currently open up to participants and recruitment began on Baronial 17, 2020. It is expected that recruitment will be complete past April 2022. As of 11 October 2021, a total of 377 participants have provided informed consent.
Abbreviations
- AE:
-
Adverse events
- CBT-I:
-
Cerebral behavioural therapy for insomnia
- CRF:
-
Case report form
- CSD-M:
-
Consensus Sleep Diary
- DSM-5:
-
Diagnostic and Statistical Manual of Mental Disorders
- EEG:
-
Electroencephalography
- GAD-7:
-
Generalised Anxiety Disorder Questionnaire (7-item)
- GDPR:
-
Full general Data Protection Regulation
- GP:
-
General practitioner
- ISI:
-
Insomnia Severity Index
- KSS:
-
Karolinska Sleepiness Calibration
- NWAK:
-
Number of awakenings
- PHQ-ix:
-
Patient Health Questionnaire (nine-particular)
- PSS:
-
Perceived Stress Scale
- SAE:
-
Serious agin outcome
- SDS-CL-25:
-
Sleep Disorders Symptom Checklist-25
- SE:
-
Slumber efficiency
- SF-36:
-
36-Item Brusque Form Survey Instrument
- SOL:
-
Sleep onset latency
- STAI:
-
Spielberger State-Trait Anxiety Inventory
- TIB:
-
Time in bed
- TST:
-
Total sleep fourth dimension
- WASO:
-
Wake subsequently sleep onset
References
-
American Psychiatric Clan. Diagnostic and Statistical Manual of Mental Disorders : DSM-five. 5th ed. Washington, DC: American Psychiatric Association; 2013. xxxvii, 943 p. p.
-
Ohayon MM. Epidemiology of indisposition: what nosotros know and what we nonetheless need to acquire. Sleep Med Rev. 2002;vi(2):97–111. https://doi.org/10.1053/smrv.2002.0186.
-
Pallesen S, Sivertsen B, Nordhus IH, Bjorvatn B. A 10-twelvemonth trend of insomnia prevalence in the developed Norwegian population. Sleep medicine. 2014;xv(2):173–ix. https://doi.org/10.1016/j.sleep.2013.x.009.
-
Daley Thou, Morin CM, LeBlanc One thousand, Gregoire JP, Savard J. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and expert sleepers. Sleep. 2009;32(one):55–64.
-
Baglioni C, Battagliese Grand, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia every bit a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(one–3):ten–nine. https://doi.org/10.1016/j.jad.2011.01.011.
-
Riemann D, Voderholzer U. Primary insomnia: a chance factor to develop depression? J Impact Disord. 2003;76(1–3):255–9. https://doi.org/10.1016/S0165-0327(02)00072-1.
-
Li M, Zhang XW, Hou WS, Tang ZY. Indisposition and risk of cardiovascular disease: a meta-analysis of cohort studies. Int J Cardiol. 2014;176(3):1044–7. https://doi.org/10.1016/j.ijcard.2014.07.284.
-
Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin Due north Am. 1987;10(four):541–53. https://doi.org/ten.1016/S0193-953X(eighteen)30532-X.
-
Spielman AJ, Nunes J, Glovinsky PB. Insomnia. Neurol Clin. 1996;14(3):513–43. https://doi.org/ten.1016/S0733-8619(05)70272-3.
-
Ellis JG, Gehrman P, Espie CA, Riemann D, Perlis ML. Acute insomnia: current conceptualizations and future directions. Sleep Med Rev. 2012;16(one):5–xiv. https://doi.org/ten.1016/j.smrv.2011.02.002.
-
Ellis JG, Perlis ML, Neale LF, Espie CA, Bastien CH. The natural history of indisposition: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46(x):1278–85. https://doi.org/10.1016/j.jpsychires.2012.07.001.
-
Perlis ML, Vargas I, Ellis JG, Grandner MA, Morales KH, Gencarelli A, et al. The Natural History of Indisposition: the incidence of acute indisposition and subsequent progression to chronic insomnia or recovery in proficient sleeper subjects. Sleep. 2020;43(half dozen):1–8.
-
Kato H, Asukai N, Miyake Y, Minakawa K, Nishiyama A. Post-traumatic symptoms among younger and elderly evacuees in the early stages post-obit the 1995 Hanshin-Awaji earthquake in Japan. Acta Psychiatrica Scandinavica. 1996;93(vi):477–81. https://doi.org/10.1111/j.1600-0447.1996.tb10680.10.
-
Mellman TA, David D, Kulick-Bong R, Hebding J, Nolan B. Sleep disturbance and its relationship to psychiatric morbidity afterwards Hurricane Andrew. Am J Psychiatr. 1995;152(11):1659–63. https://doi.org/10.1176/ajp.152.11.1659.
-
Askenasy JJ, Lewin I. The touch of missile warfare on self-reported sleep quality. Function 1. Sleep. 1996;19(1):47–51. https://doi.org/ten.1093/sleep/19.1.47.
-
Seelig Advertizing, Jacobson IG, Smith B, Hooper TI, Boyko EJ, Gackstetter GD, et al. Sleep patterns before, during, and afterwards deployment to Iraq and Afghanistan. Slumber. 2010;33(12):1615–22. https://doi.org/10.1093/sleep/33.12.1615.
-
Jahrami H, BaHammam AS, Bragazzi NL, Saif Z, Faris M, Vitiello MV. Sleep problems during the COVID-19 pandemic past population: a systematic review and meta-assay. J Clin Sleep Med. 2021;17(2):299–313. https://doi.org/10.5664/jcsm.8930.
-
Wang J, Gong Y, Chen Z, Wu J, Feng J, Yan S, et al. Sleep disturbances among Chinese residents during the Coronavirus Disease 2019 outbreak and associated factors. Slumber medicine. 2020;74:199–203. https://doi.org/10.1016/j.sleep.2020.08.002.
-
Casagrande M, Favieri F, Tambelli R, Forte G. The enemy who sealed the world: furnishings quarantine due to the COVID-19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep medicine. 2020;75:12–20. https://doi.org/10.1016/j.sleep.2020.05.011.
-
Ellis JG, Cushing T, Germain A. Treating astute insomnia: a randomized controlled trial of a "single-shot" of Cognitive Behavioral Therapy for Insomnia. Sleep. 2015;38(6):971–eight. https://doi.org/x.5665/sleep.4752.
-
Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, et al. European guideline for the diagnosis and treatment of insomnia. J Slumber Res. 2017;26(6):675–700. https://doi.org/ten.1111/jsr.12594.
-
Stranks EK, Crowe SF. The acute cerebral effects of zopiclone, zolpidem, zaleplon, and eszopiclone: a systematic review and meta-assay. J Clin Exp Neuropsychol. 2014;36(vii):691–700. https://doi.org/10.1080/13803395.2014.928268.
-
Kripke DF, Langer RD, Kline LE. Hypnotics' association with mortality or cancer: a matched cohort written report. BMJ open. 2012;2(i):one–8.
-
Siriwardena AN, Qureshi MZ, Dyas JV, Middleton H, Orner R. Magic bullets for insomnia? Patients' employ and experiences of newer (Z drugs) versus older (benzodiazepine) hypnotics for sleep problems in master care. British Journal of General Practice. 2008;58(551):417–22. https://doi.org/ten.3399/bjgp08X299290.
-
Glass J, Lanctot KL, Herrmann North, Sproule BA, Busto UE. Sedative hypnotics in older people with indisposition: meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169. https://doi.org/10.1136/bmj.38623.768588.47.
-
Randall C, Nowakowski S, Ellis JG. Managing acute insomnia in prison: evaluation of a "ane-shot" Cognitive Behavioral Therapy for Insomnia (CBT-I) intervention. Behav Slumber Med. 2019;17(6):827–36. https://doi.org/10.1080/15402002.2018.1518227.
-
Boullin P, Ellwood C, Ellis JG. Grouping vs. private treatment for astute indisposition: a pilot study evaluating a "one-shot" treatment strategy. Brain Sci. 2016;vii(1):1-x.
-
van der Zweerde T, Lancee J, Ida Luik A, van Straten A. Internet-delivered Cerebral Behavioral Therapy for Indisposition: tailoring Cognitive Behavioral Therapy for Insomnia for patients with chronic insomnia. Slumber Med Clin. 2019;14(iii):301–fifteen. https://doi.org/10.1016/j.jsmc.2019.04.002.
-
Zachariae R, Lyby MS, Ritterband LM, O'Toole MS. Efficacy of internet-delivered cognitive-behavioral therapy for indisposition - a systematic review and meta-assay of randomized controlled trials. Sleep Med Rev. 2016;xxx:i–10. https://doi.org/10.1016/j.smrv.2015.ten.004.
-
Ellis JG, Perlis ML, Espie CA, Grandner MA, Bastien CH, Barclay NL, et al. The Natural History of Insomnia: predisposing, precipitating, coping and perpetuating factors over the early developmental course of indisposition. Sleep. 2021;44(9). https://doi.org/10.1093/sleep/zsab095.
-
Perlis ML, Morales KH, Vargas I, Posner DA, Grandner MA, Muench AL, et al. The natural history of insomnia: does sleep extension differentiate between those that practice and do not develop chronic insomnia? J Sleep Res. 2021;xxx(5):e13342.
-
Harvey AG, Payne Due south. The management of unwanted pre-sleep thoughts in insomnia: distraction with imagery versus general distraction. Behav Res Ther. 2002;40(3):267–77. https://doi.org/10.1016/S0005-7967(01)00012-two.
-
Espie CA. Overcoming indisposition: a self-assist guide using cognitive behavioral techniques. London: Robinson; 2006.
-
Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index equally an consequence measure for insomnia research. Sleep Med. 2001;ii(4):297–307. https://doi.org/ten.1016/S1389-9457(00)00065-4.
-
Spitzer RL, Kroenke Grand, Williams JB, Lowe B. A brief measure for assessing generalized feet disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–vii. https://doi.org/10.1001/archinte.166.10.1092.
-
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;sixteen(9):606–13. https://doi.org/x.1046/j.1525-1497.2001.016009606.x.
-
Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal Ad, Lichstein KL, et al. The consensus sleep diary: standardizing prospective sleep cocky-monitoring. Slumber. 2012;35(2):287–302. https://doi.org/10.5665/sleep.1642.
-
Faul F, Erdfelder Due east, Buchner A, Lang AG. Statistical ability analyses using K*Power 3.1: tests for correlation and regression analyses. Behavior research methods. 2009;41(iv):1149–lx. https://doi.org/10.3758/BRM.41.4.1149.
-
Perusse Advertising, De Koninck J, Pedneault-Drolet One thousand, Ellis JG, Bastien CH. REM dream activity of insomnia sufferers: a systematic comparison with good sleepers. Sleep medicine. 2016;xx:147–54. https://doi.org/10.1016/j.sleep.2015.08.007.
-
Schredl M, Schafer Thou, Weber B, Heuser I. Dreaming and indisposition: dream recall and dream content of patients with insomnia. J Sleep Res. 1998;7(3):191–8. https://doi.org/10.1046/j.1365-2869.1998.00113.ten.
-
Bastien CH, Vallieres A, Morin CM. Precipitating factors of insomnia. Behav Sleep Med. 2004;2(1):fifty–62. https://doi.org/10.1207/s15402010bsm0201_5.
-
Ware JE Jr, Sherbourne CD. The MOS 36-item short-grade health survey (SF-36). I. Conceptual framework and item choice. Med Care. 1992;xxx(6):473–83. https://doi.org/ten.1097/00005650-199206000-00002.
-
Jin W, Yu H. A study of the reliability and validity of SF-36 scale on evaluating health of population. Chin Health Resour. 2012;xv:265–7.
-
Klingman KJ, Jungquist CR, Perlis ML. Introducing the Sleep Disorders Symptom Checklist-25: a main care friendly and comprehensive screener for slumber disorders. Sleep Med Res. 2017;viii(1):17–25. https://doi.org/10.17241/smr.2017.00010.
-
Morin CM, Belleville Thousand, Belanger L, Ivers H. The Indisposition Severity Index: psychometric indicators to notice indisposition cases and evaluate handling response. Sleep. 2011;34(5):601–8. https://doi.org/10.1093/sleep/34.5.601.
-
Åkerstedt T, Gillberg Chiliad. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1-2):29–37. https://doi.org/10.3109/00207459008994241.
-
Kaida K, Takahashi Grand, Åkerstedt T, Nakata A, Otsuka Y, Haratani T, et al. Validation of the Karolinska sleepiness scale confronting performance and EEG variables. Clin Neurophysiol. 2006;117(vii):1574–81. https://doi.org/10.1016/j.clinph.2006.03.011.
-
Schredl Thousand, Berres S, Klingauf A, Schellhaas S, Göritz AS. The Mannheim Dream questionnaire (MADRE): Retest reliability, age and gender furnishings. International Periodical of Dream Research. 2014;7(2):141–7.
-
Marteau TM, Bekker H. The evolution of a six-particular short-course of the state scale of the Spielberger State-Trait Feet Inventory (STAI). Br J Clin Psychol. 1992;31(3):301–half-dozen. https://doi.org/10.1111/j.2044-8260.1992.tb00997.x.
-
Löwe B, Decker O, Müller South, Brähler E, Schellberg D, Herzog Due west, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Intendance. 2008;46(3):266–74. https://doi.org/10.1097/MLR.0b013e318160d093.
-
Martin A, Rief W, Klaiberg A, Braehler Eastward. Validity of the brief patient health questionnaire mood calibration (PHQ-9) in the general population. Gen Hospital Psychiatry. 2006;28(ane):71–7. https://doi.org/x.1016/j.genhosppsych.2005.07.003.
-
Cohen South, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. https://doi.org/10.2307/2136404.
-
Siqueira Reis R, Ferreira Hino AA. Romélio Rodriguez Añez C. Perceived stress scale: reliability and validity written report in Brazil. J Wellness Psychol. 2010;fifteen(1):107–fourteen. https://doi.org/x.1177/1359105309346343.
-
Lee E-H. Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res. 2012;half-dozen(4):121–7. https://doi.org/10.1016/j.anr.2012.08.004.
-
Weiss DS. The Impact of Consequence Scale: Revised. In: Wilson JP, Tang CS-K, editors. Cantankerous-cultural assessment of psychological trauma and PTSD. Boston: Springer Us; 2007. p. 219–38. https://doi.org/10.1007/978-0-387-70990-1_10.
-
Duncan LA, Schaller M, Park JH. Perceived vulnerability to affliction: evolution and validation of a 15-detail cocky-report instrument. Personality Private Differences. 2009;47(half-dozen):541–6. https://doi.org/ten.1016/j.paid.2009.05.001.
-
Luik AI, van der Zweerde T, van Straten A, Lancee J. Digital delivery of cognitive behavioral therapy for indisposition. Curr Psychiatry Rep. 2019;21(7):fifty. https://doi.org/10.1007/s11920-019-1041-0.
-
Weiner Fifty, Berna F, Nourry N, Severac F, Vidailhet P, Mengin AC. Efficacy of an online cognitive behavioral therapy program developed for healthcare workers during the COVID-nineteen pandemic: the REduction of STress (REST) written report protocol for a randomized controlled trial. Trials. 2020;21(1):870. https://doi.org/x.1186/s13063-020-04772-7.
-
Webb CA, Rosso IM, Rauch SL. Internet-based cognitive-behavioral therapy for depression: current progress and futurity directions. Harv Rev Psychiatry. 2017;25(3):114–22. https://doi.org/10.1097/HRP.0000000000000139.
-
Spek 5, Cuijpers P, Nyklicek I, Riper H, Keyzer J, Pop V. Internet-based cerebral behaviour therapy for symptoms of depression and anxiety: a meta-assay. Psychol Med. 2007;37(iii):319–28. https://doi.org/10.1017/S0033291706008944.
-
Lancee J, van den Tour J, Sorbi MJ, van Straten A. Motivational support provided via electronic mail improves the effectiveness of internet-delivered cocky-help treatment for indisposition: a randomized trial. Behav Res Ther. 2013;51(12):797–805. https://doi.org/x.1016/j.brat.2013.09.004.
-
Higgins JS, J, Page MJ, Elbers RG, Sterne JAC. Assessing hazard of bias in a randomized trial. 2021. In: Cochrane Handbook for Systematic Reviews of Interventions [Net]. Cochrane.
Acknowledgements
OS was financially supported by a Northumbria University Graduate Futures Undergraduate Research Internship. We would also like to give thanks Will Atkinson for projection help. We are besides very grateful to Professor Thomas Five. Pollet for advice regarding the statistical analysis.
Authors' contributions {31b}
GE and JE designed the study. PA-M and NS too contributed to the design of the study and development of the written report protocol. OS and GE drafted the manuscript. All authors (GE, OS, PA-Thou, NS and JE) have contributed to the disquisitional analysis and revisions of the manuscript and accept read and canonical the final manuscript.
Funding {four}
This written report is financially supported by Northumbria Academy. The funder has no directly function in the design of the written report, in the collection, analysis and interpretation of data, or in writing the manuscript.
Author information
Affiliations
Corresponding author
Ethics declarations
Ethics blessing and consent to participate {24}
This trial received ethical approval from the Northumbria University ethics commission (Reference number: 23377) on 9 April 2020. Informed consent will be obtained electronically from all participants.
Consent for publication {32}
Not applicable.
Competing interests {28}
No members of the report squad have any competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in whatever medium or format, as long every bit you give appropriate credit to the original author(south) and the source, provide a link to the Creative Commons licence, and betoken if changes were made. The images or other third party textile in this article are included in the commodity's Creative Commons licence, unless indicated otherwise in a credit line to the material. If textile is not included in the commodity'southward Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted utilise, you lot volition demand to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the information fabricated available in this article, unless otherwise stated in a credit line to the information.
Reprints and Permissions
Near this commodity
Cite this article
Sawdon, O.L., Elder, G.J., Santhi, N. et al. Testing an early online intervention for the handling of disturbed sleep during the COVID-xix pandemic in self-reported good and poor sleepers (Slumber COVID-19): study protocol for a randomised controlled trial. Trials 22, 913 (2021). https://doi.org/10.1186/s13063-021-05888-0
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/x.1186/s13063-021-05888-0
Keywords
- Randomised controlled trial
- Slumber disturbances
- Acute insomnia
- COVID-xix
- Stress
- Online intervention
Source: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-021-05888-0
0 Response to "Sleepers Assignment Read and Respond # 06"
Post a Comment